A peptide bond (amide bond) is a covalent chemical bond formed between twomolecules when the carboxyl group of one molecule reacts with the amino groupof the other molecule, causing the release of a molecule of water (H2O), hence the process is a dehydration synthesis reaction (also known as a condensation reaction), and usually occurs between amino acids. The resulting C(O)NH bond is called a peptide bond, and the resulting molecule is an amide. The four-atom functional group -C(=O)NH- is called a peptide link. Polypeptides and proteinsare chains of amino acids held together by peptide bonds, as is the backbone ofPNA.
A peptide bond can be broken by amide hydrolysis (the adding of water). The peptide bonds in proteins are metastable, meaning that in the presence of water they will break spontaneously, releasing 2-4 kcal/mol [1] of free energy, but this process is extremely slow. In living organisms, the process is facilitated by enzymes. Living organisms also employ enzymes to form peptide bonds; this process requires free energy. The wavelength of absorbance for a peptide bond is 190-230 nm
The Peptide BondFormation of the peptide bond
The molecules must be orientated so that the carboxylic acid group of one can react with the amine group of the other. For example, two amino acids (glycine) combining through the formation of a peptide bond to form a dipeptide.

Any number of amino acids can be joined together in chains of 50 amino acids called peptides, 50-100 amino acids called polypeptides, and over 100 amino acids called proteins. A number of hormones, antibiotics, antitumor agents and neurotransmitters are peptides (proteins).
A peptide bond can be broken down by hydrolysis (the adding of water). The peptide bonds that are formed within proteins have a tendency to break spontaneously when subjected to the presence of water (metastable bonds) releasing about 10 kJ/mol of free energy. This process, however, is very slow. Living organisms use enzymes to broken down or to form peptide bonds. The wavelength of absorbance for a peptide bond is 190-230 nm.
Structure of the Peptide Bond
X-ray diffraction studies of crystals of small peptides by Linus Pauling and R. B. Corey indicated that the peptide bond is rigid, and planer. Pauling pointed out that this is largely a consequence of the resonance interaction of the amide, or the ability of the amide nitrogen to delocalize its lone pair of electrons onto the carbonyl oxygen.
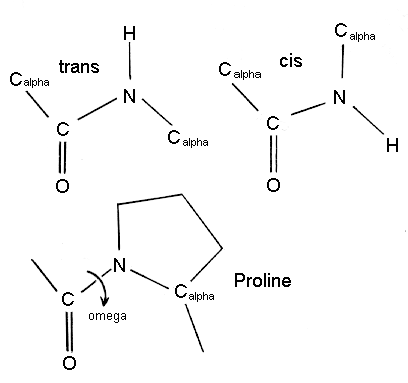
Because of this resonance, the C=O bond is actually longer than normal carbonyl bonds, and the N–C bond of the peptide bond is shorter than the N–Cα bond. Notice that the carbonyl oxygen and amide hydrogen are in a trans configuration, as opposed to a cis configuration. This configuration is energetically more favorable because of possible steric interactions in the other.
The Polarity of the Peptide Bond
The peptide bond is usually portrayed as a single bond between the carbonyl carbon and the amide nitrogen. Normally, this should allow free rotation about than bond. However, notice that the nitrogen has a lone pair of electrons, which are adjacent to a carbon-oxygen bond. Therefore, a reasonable resonance structure can be draw with a double bond linking the carbon and nitrogen, and which result in a negative charge on the oxygen and a positive charge on the nitrogen.
The resonance structure prevents rotation around the peptide bond. The real structure of course is a weighted hybrid of these two structures. Therefore, the question is how significant is the resonance structure in depicting the true electron distribution. It is know that the peptide bond has approximately 40% double-bond character and therefore it is rigid.
Charges give the peptide bond a permanent dipole. Because of the resonance, the oxygen has a -0.28 charge, while the nitrogen bears a +0.28 charge.
| ||

Click for larger image |
| |

|
|

2 comments:
This content is written very well. Your use of formatting when making your points makes your observations very clear and easy to understand. Thank you. buy peptide hormones
AdTx1 is a 65 amino-acid peptide and a selective, high affinity, non-competitive α1A adrenoceptor antagonist (Ki = 0.35 nM). It exhibits no significant activity against a range of other GPCRs, including α2A, β1 and β2 adrenoceptors. AdTx1 is used as a potent relaxant of smooth muscles. AdTx1
Post a Comment